Scanning Ion Conductance Microscopy
Scanning probe microscopy is based on scanning a fine probe over a surface, using the local interaction between the probe and surface to maintain constant probe - sample distance and hence obtain a high resolution image of the sample. While atomic resolution is possible in the physical sciences using scanning tunneling microscopy and atomic force microscopy and these methods are now widely used, the application of scanning probe microscopy to living cells in the field of biological sciences has been much more limited. This is due to the difficulties in imaging the soft and responsive cell surface.
SICM Principles of Operation

The SICM consists of a glass micropipette probe filled with electrolyte lowered into a bath of electrolyte (see diagram) toward an insulating, for ions, surface of the sample. As the tip of the micropipette approaches the sample, the ion conductance reduces because the gap that ions can flow through is decreased. Changes of the ion current are measured by an ion current amplifier, and are used as a feedback input signal by scanner control unit to keep the distance between pipette tip and sample constant by applying corresponding voltages to the Z-piezo drive during the scanning procedure. Therefore, the path of the tip follows the topography of the surface.
SICM Applications
We have recently pioneered the development of an array of new and powerful biophysical tools that allow quantitative measurements and non-invasive functional imaging of single protein molecules in living cells. Scanning ion conductance microscopy and a battery of associated innovative methods are unique among current imaging techniques (see left figure), not only in spatial resolution of living and functioning cells, but also in the rich combination of imaging with other functional and dynamical interrogation methods. These methods, crucially, will facilitate the study of integrated nano-behaviour in living cells in health and disease.
The family of techniques we have developed is shown in the figures below.
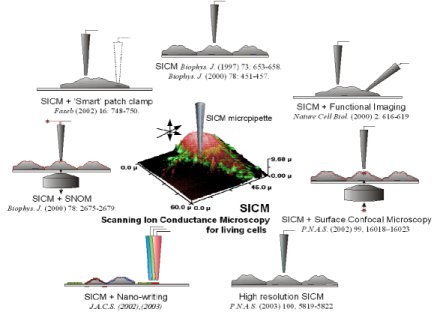
The methodologies are applicable to a wide variety of different cell types and tissues and we have applied these methods to neuro, cardiac and sperm physiology, endocrinology and virology. Our key achievements are:
- The development of a unique non-contact system for imaging living cell surfaces and their dynamics down to the level of individual protein complexes. The method is based on a scanned nanopipette, using ion conductance feedback to maintain constant pipette-sample distance. We can interrogate unfixed, unstained, living cells with high resolution. This enabled us to:
- · Imaging protein complexes on a live cell.
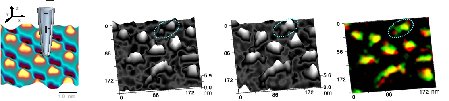
- · Determine the dynamics of microvilli and how they can be used as building blocks to assemble more complex structures.
- · High resolution Hopping mode imaging.
.jpg)
-
· Develop an in-vitro model of rhythm disturbance in heart and study bile acid effects on cardiac myocyte function.
